PH of a strong acidbase solution. PH -log 10 H H 10 -pH.

3 Link Save.
Calculate ph on calculator. With this pH calculator you can determine the pH of a solution in a few ways. It can convert pH to H as well as calculate pH from the ionization constant and concentration. PH is an essential factor in chemistry medicine and daily lifeRead the text below to find out what is the pH scale and the pH formulaIn the end we will also explain how to calculate pH with an easy step-by-step.
PH Calculator is a free online tool that displays the pH value for the given chemical solution. BYJUS online pH calculator tool makes the calculation faster and it displays the pH measurement in a fraction of seconds. How to Use pH Calculator.
The procedure to use the pH calculator is as follows. Enter the chemical solution name and its concentration value in the respective input. Given pH Calculator is helpful to know the pH value of a chemical solution.
Just provide the input ie concentration and chemical formula and hit on the calculate button to get the pH scale as output along with an explanation. Free online pH calculator for acids bases and salts. Calculations are based on hydrochemistry program PhreeqC.
Add 1 2 or 3 reactants to water. Equilibrium with atmospheric CO 2 Open System Clear All. Acidic Reactants 90 NH 4 2 Cr 2 O 7.
Ammonium dichromate NH 4 2 SeO 4. Ammonium selenate NH 4. This calculated value is fundamental in chemistry because it has implications for industrial pharmaceutical and other commercial applications such as food and wine.
In addition pH is a measurement of acidity or alkalinity which can be useful for scientific and environmental applications. It is a fundamental parameter used to understand the properties of soil and water. PH is also closely.
Calculate the pH of a mixture for the solutions given belowEnter initial concentration and volume for each solution andor. Add acidsbases not appearing in the table. Enter concentration volume and pK for each of the added solutions.
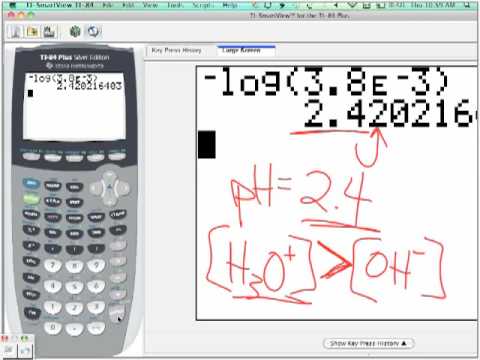
If given concentrations are meant to be those inside the final mixture keep all volumes to zero. Compound Concentration molL Volume L HCl. Calculating the pH and ion concentrations on a Calculator TI-83 or similar I.
Given ion concentration find the pH. To obtain the pH of a solution you must compute the negative log of the hydrogen ion concentration H Step 1. Enter negative value - Step 2.
Enter Log LOG Step 3. Enter ion concentration value -Log value. This online calculator calculates pH of the solution given solute formula and solution molarity.
The solute is assumed to be either strong acid or strong base. Articles that describe this calculator. PH of a solution calculator.
PH of a strong acidbase solution. Digits after the decimal point. 3 Link Save.
PH 1 is the measure of acidity of a solution. PH measurement is derived from the measure of hydronium ion concentration. Since the ion concentration is small in the solution it is converted by the equation above to pH the equation above is a derivation of a equation that can be found here and compared against a scale pH scale ranges from 0 to 14 0 being acidic and 14 being basic.
This tool allows the calculation of the pH values of diluted bases and acids. Please insert three values the fourth will be calculated. You can choose which value you want to leave blank.
As a neutral dilution liquid pH value 7 water is estimated. The calculated value is a good estimation. Calculating pH without a calculator Published by chelseaemyers on June 6 2019 June 6 2019 If youve drawn up a schedule to study for the MCAT this summer its an easy bet that youve dedicated some serious time to general chemistry.
Build your own widget. Free Pool pH Adjustment Calculator. Adjust your pools pH level.
This tool will tell you what you need to do in order to adjust your pools pH level. Please enter your pools current pH level and your desired pH level below and then click the Calculate button. You are currently using US.
Click here to switch to metric units. Free Pool Calculator. Learn how to calculate the pH of a concentration.
Calculator input also given. How to Calculate pH and H The equilibrium equation yields the following formula for pH. PH -log 10 H H 10 -pH.
In other words pH is the negative log of the molar hydrogen ion concentration or the molar hydrogen ion concentration equals 10 to the power of the negative pH value. Its easy to do this calculation on any scientific. Calculating pH of Acids and Bases.
Calculation of pH is simple when there is a 1 times 10textpower problem. However in real life this is rarely the situation. If the coefficient is not equal to 1 a calculator must be used to find the pH.
For example the pH of a solution with left ceH right 23 times 10-5. Define pH in an equation. The pH scale is calculated by a negative logarithm.
A negative logarithm of base b is simply how many times a number must be divided by b to reach 1. The pH equation can be seen as follows. PH -log 10 H 3 O.
The equation can sometimes be seen as pH -log 10 H Know that whether the equation has H 3 O or H they are the same. Calculator for the comparison of two pH-values of acids and bases. The pH-value tells how acidic or alkaline an aqueous solution is.
With a value of 7 it is neutral that is eg. The value for pure water. Here the amount of H 3 O ions is equal to the amount of HO ions.
For smaller values the solution is acidic more.